Water Methanol Mixture Viscosity

The results were compared with the available experimental data as well as some theoretical models.
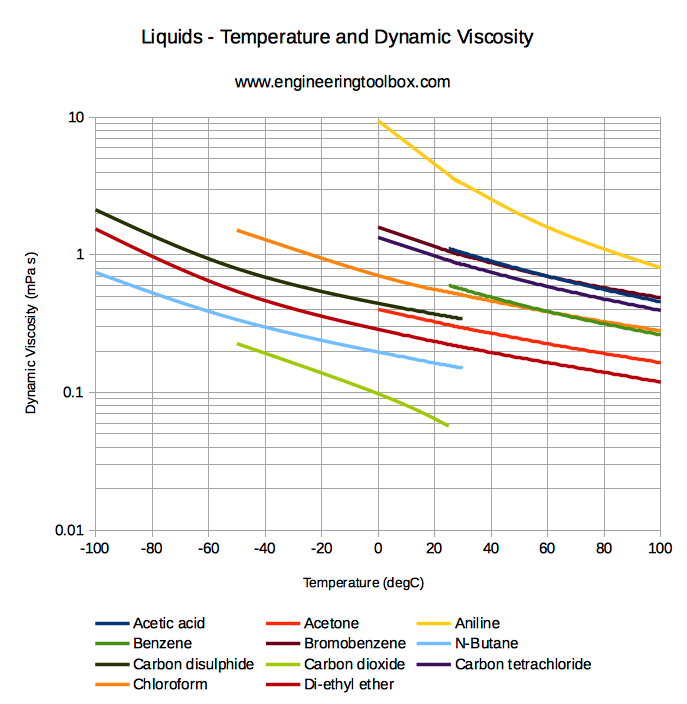
Water methanol mixture viscosity. In this method a viscosity blending number vbn of each component is first calculated and then used to determine the vbn of the liquid mixture as shown below. Generaly the viscosity decreases with increase in concentration of the organic modifier acetonitrile or methanol respectively. The kinematic viscosity of the mixture can then be estimated using the. Unusual behaviour probably not fully understood.
Also dynamic properties are. Viscosity is important properties for engineering such that designing chemical plants requires precise information about the viscosity of solutions. Viscosity of ethanol water mixture viscosity is the physical quantity specifying the flowing properties of liquids. Viscosity measurements have been made to the solutions of dodecyltrimethylammonium bromide dtab and cetyltrimethylammonium bromide ctab in 0 10 0 20 0 30 and 0 40 volume fractions of methanol in methanol water mixed solvent media at 298 15 308 15 318 15 and 323 25 k.
Critical micelle concentration cmc values have been determined. Overall indicating a good agreement. Further as you can see from the following figures the viscosity decrease with higher temperature which forces further research in the field of. Refutas 2000 proposed a method by which the kinematic viscosity of a mixture of two or more liquids.
Viscosity itself is physically expressed as transportation phenomenon of momentum in flowing liquids. At different compositions but with the same viscosity molecular properties are different as measured by probe molecules having different rotational diffusion times. Begingroup alcohol water mixtures have v. The solution behavior of the mixtures is discussed in terms of the variation of the excess viscosity and excess volume with.
Viscosity of two component mixtures viscosity of water ethanol and water isopropyl alcohol mixtures. Prediction of self diffusion coefficient and shear viscosity of water and its binary mixtures with methanol and ethanol by molecular simulation. In this study some properties of the methanol water mixture such as diffusivity density viscosity and hydrogen bonding were calculated at different temperatures and atmospheric pressure using molecular dynamics simulations mds. However these compounds behave like non.
Following tables and plots show dependence of the viscosity for a acetonitrile water and a methanol water mixtures at different composition of the binary solvent and temperature. The experimental data of viscosity and density for six binary mixtures of water methanol or ethanol with an ionic liquid 1 butyl 3 methylimidazolium dimethylphosphate bmim dmp or 1 ethyl 3 methylimidazolium dimethylphosphate emim dmp were measured in the temperature range of 293 15 to 333 15 k at atmospheric pressure using a viscometer and densimeter. The journal of chemical physics 2011 134 7 074508. The vbn of the liquid mixture is then calculated as follows.
This shows the useful and. Water alcohol mixtures are widely used in chemical engineering applications such as solvents for gums resins lacquers varnishes and dyes.