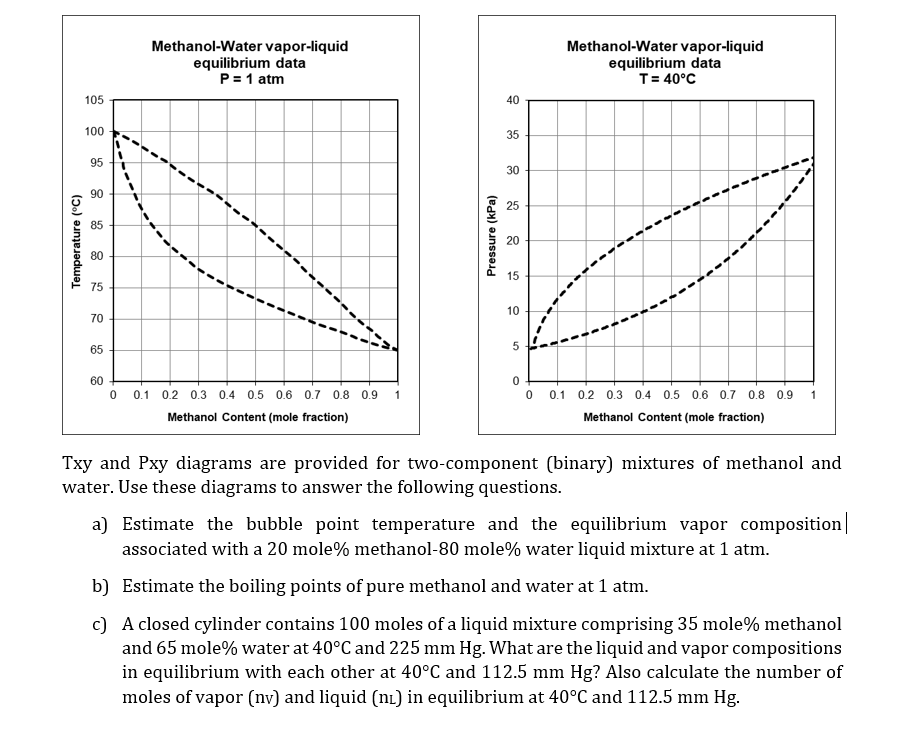
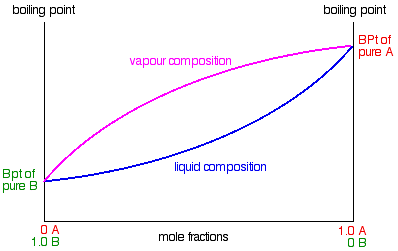
Water Methanol Mixture Boiling Point
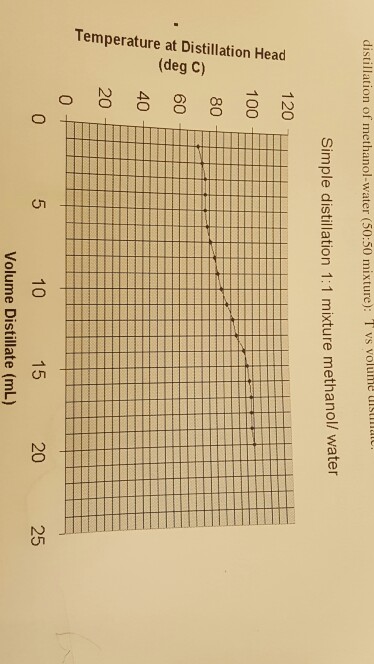
This variation is depicted graphically in figure 4.
Water methanol mixture boiling point. 6 1929 1544 1549 vapor liquid equilibrium data set 467. Freezing points of methanol. Then once all the metha. A laboratory experiment on the boiling point curves of non azeotropic binary mixtures.
173 1 170 6 168 1 165 6 atmospheric pressure. Boiling point of water. 212 0 208 9 206 1 203 0 boiling point of ethanol. The composition of this bubble is different to the composition of the liquid.
Insert thermometer c position instrument over flame. Binary mixtures methanol water vle t x y 60. A table of several predicted and subsequent experimental boiling point differences for methanol mixtures can be found in figure 6 assuming a boiling point for pure methanol of 64 7 ºc and a kb of 83 for pure methanol. Freezing points of methanol water solutions by weight by volume 10 0 temperature c novosolution ca 10 20 30 40 50 60 70 80 90 100 110 120 130 100 90 80 70 60 50 40 30 20 10 concentration of methanol novosolution calculates methanol concentration using the by weight method.
A chart that shows the latent. A chart that demonstrates the boiling and flash points for methanol and methanol water solutions. For non azeotropic mixtures the boiling point of each component of the mixture will remain the same. A chart that shows the relative density of water methanol mixes.
A chart that shows the relative freezing points for methanol and methanol water solutions. 78 4 77 0 75 6 74 2 boiling point of ethanol. As is shown in table 1 the jakob number varies by an order of magnitude over the range of fluids tested for a given channel temperature. Boiling point of the mixture depends on the relative amounts of the two components present a mixture containing 30 meth 70 h2o has a b p.
The data include the composition of a mixture by weight in binary azeotropes when only one fraction is given it is the fraction of the second component the boiling point b p of a component the boiling point of a mixture and the specific gravity of the mixture. Determine boiling point of water add approximately 30 ml of deionized water to boiling chamber a there is no need to add cold tap water to condenser d at this time. 6 1929 1544 1549 j chem educ. We call this the bubble point.
This page contains tables of azeotrope data for various binary and ternary mixtures of solvents. That s how distillation works. 100 0 98 3 96 7 95 0 boiling point of water. When thermometer reaches a stable point allow 15 30 sec for minor fluctuations to occur.
Highlights the need to understand the boiling point for the methanol water mixture since it will also vary with varying molar fraction in the binary mixture. As is apparent even a mixture of 50 percent methanol would force methanol s boiling point above the boiling point of water by the bpee figure 5.