Isolation Of Caffeine From Tea Bag Lab Report
Using the proper extraction methods the caffeine within a tea bag could potentially be isolated to yield a pure solid.
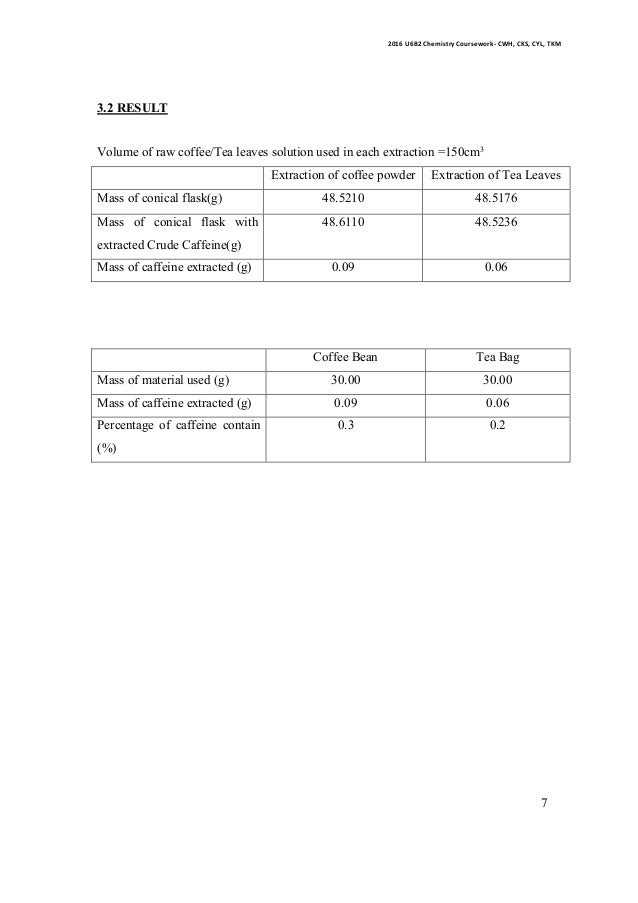
Isolation of caffeine from tea bag lab report. This suggested that it contained impurity. The tea bag was estimated to have 0 11 grams of caffeine which is the initial amount used to calculate extraction yield. The mass of this solid would reflect the actual yield of caffeine in the tea. Sublimation was used to extract pure caffeine from the impure.
The extraction yield was calculated by dividing the final amount 0 081 grams by the initial. Standard tea bags contain 2 00 0 05 g of tea leaves along with approximately 55 mg of caffeine. 13 of pure caffeine was recovered after sublimation. Caffeine is one of the main extracted substances found in a tea solution and it can make up about 5 of the weight of a tea bag.
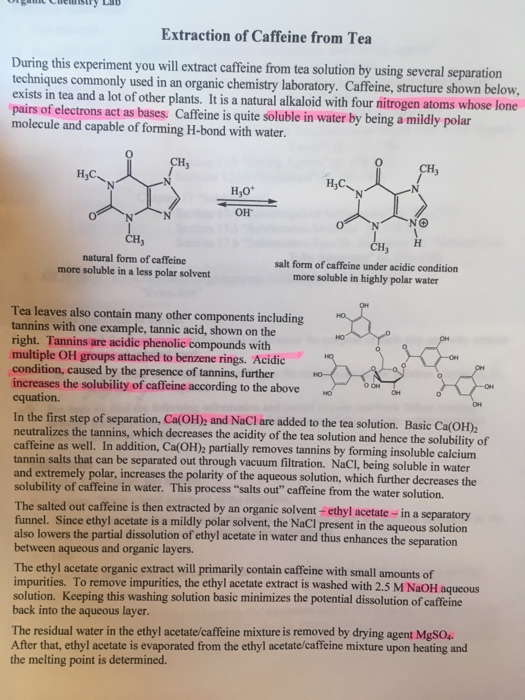
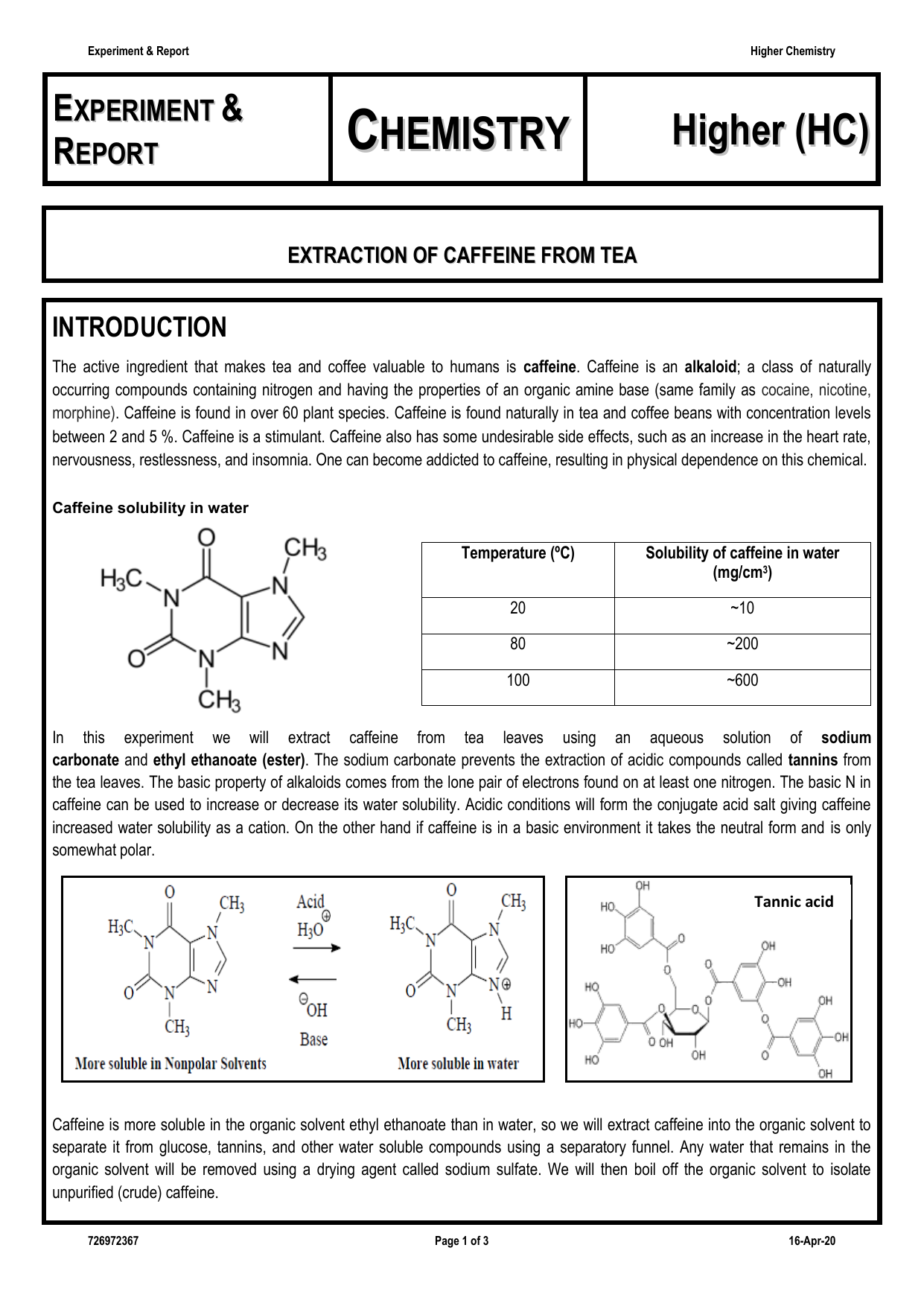
The purpose of this experiment was extraction of caffeine from dry tea leaves. Tea leaves were weighed having 8 5333 grams. In order to extract caffeine from tea several methods are used. Caffeine from tea leaves caffeine must be present as the free base amrita 2013.
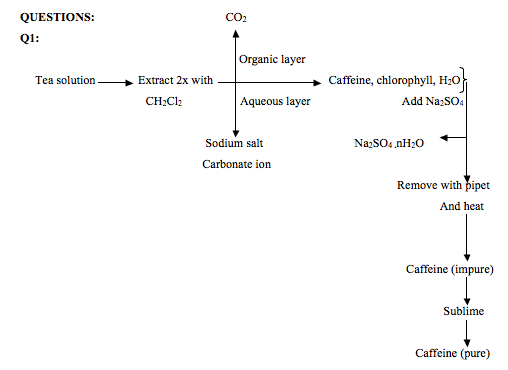
Extraction of caffeine from tea bags abstract caffeine extraction from the commercial tea leaves lipton yellow label tea that was done is multiple extraction. In order to do so the above mentioned acidic substances must remain water soluble. The water soluble compounds of caffeine were separated from the tea bag with boiling water and then extracted into an organic solvent to. The tea bag yielded 0 031g of crude caffeine which is about 1 3 of the mass of tea in one tea bag.
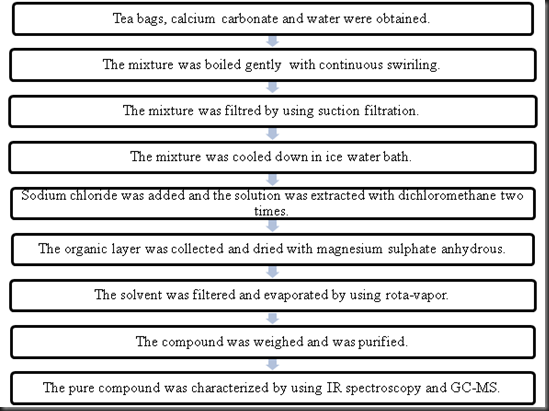
During the experiment a tea bag was heated in boiling water for about 15 minutes. Report for experiment 7. 0 081 grams of caffeine were recovered after extraction. Sodium carbonate and hot water were added to the tea bags and was let to stand for about 7 minutes in order to bring the caffeine molecules out of the tea bags and into the aqueous solution.
The impure caffeine had a melting point range of 223 6 229 3 0 c. Isolation of caffeine section 12 02 nattanit trakullapphan nam narissara pracharktam nik thaksaporn sirichanyaphong may abstract. First a solid liquid extraction must take place in order to get the solid natural product into the liquid solvent. The resulting mixture was collected for the extraction of caffeine.
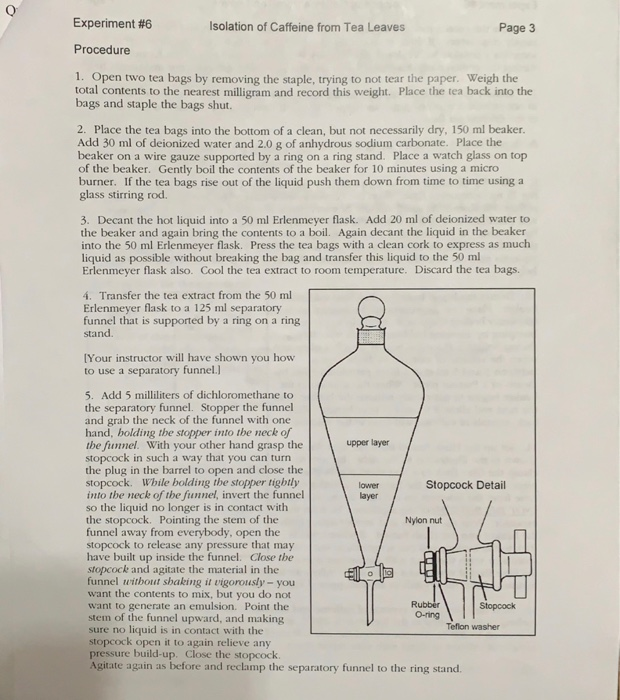
In this experiment caffeine was extracted and sublimed from a tea bag. 4 tea bags were used in the experiment. Caffeine can be extracted from tea by its ability to be better dissolved in dichloromethane than water. The aqueous tea extract was transferred in a seperatory funnel mixed with dcm 20ml done thrice.
The purpose of this experiment was to determine the yield percent recovery and melting point of caffeine isolated from tea bag. 10 4 13 isolation of caffeine from tea objective.